

Share the latest information



Shanghai, February 29, 2024 - Novamab, a biotechnology company dedicated to the research and development of innovative VHH antibody drugs, announced the research progress on the development and scaling up of anti TSLP VHH antibody (Nb3341) based on the Pichia pastoris expression system. The related achievements were published online in the journal “Protein Expression and Purification” under the title "High performance production process development and scale-up of an anti-TSLP VHH antibody", with Dr. Yakun Wan as the corresponding author.

VHH antibodies are a class of genetically engineered antibodies with enormous potential application value in the field of biotechnology such as threpay and diagnosis. This study established a fermentation culture and protein purification method for anti-TSLP VHH antibody (Nb3341) in Pichia pastoris expression system, and successfully scaled up the production scale of anti-TSLP VHH antibody (Nb3341) to a 100 L. The study determined the small-scale process through PB experiments, fermentation process optimization, and purification filler screening. After process scale-up, the expression level of Nb3341 at the 100 L scale was 22.97 g/L, with a crude SEC-HPLC purity of 98.7%, a cation exchange chromatography (AEX) HPLC purity of 95.7%, t a host cell protein (HCP) content of 4 ppm, and a host cell DNA (HCD) residual amount of 1 pg/mg. The results of the 100 L scale-up experiment were consistent with the results of the 7 L small-scale process development, further proving the feasibility and stability of this method. This study provides an efficient and stable pathway for the transformation of VHH antibodies from laboratory scale to commercial production scale. Based on the stable genetic characteristics and process reproducibility of Pichia pastoris, it provides a solid research foundation for achieving larger scale commercial production in the future.
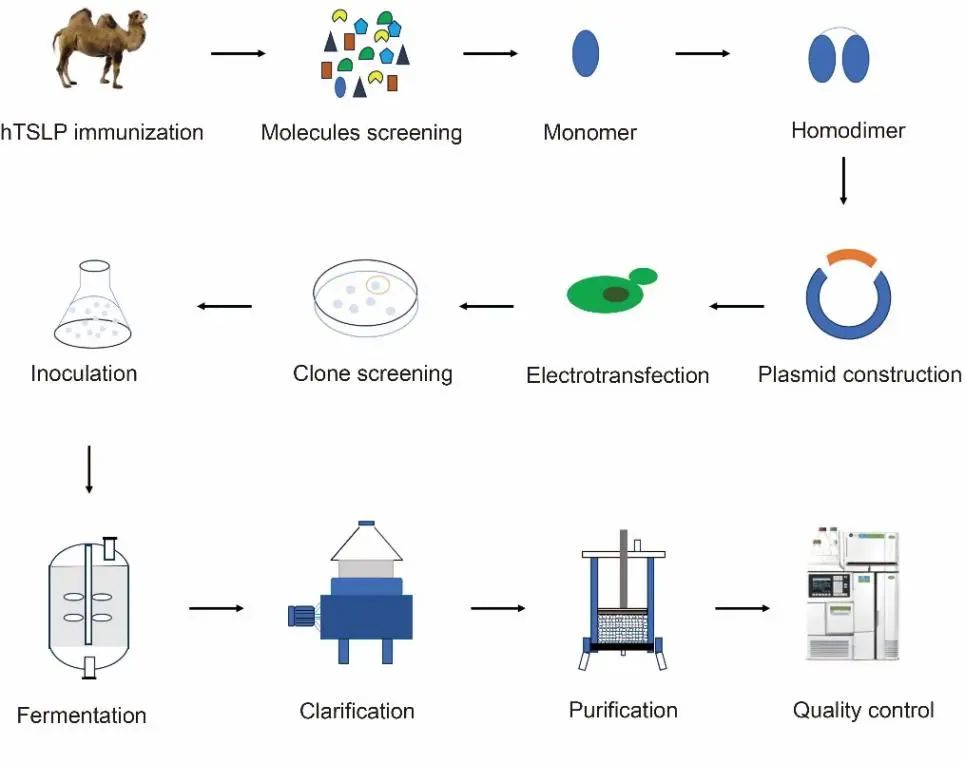
Figure 1 Flow chart of VHH antibody immunization, screening, expression, and purification
About TSLP
Thymic stromal lymphopoietin (TSLP) is an epithelial cell-derived cytokine that plays a crucial role in regulating type 2 inflammatory responses at barrier surfaces such as the skin, respiratory tract, and gastrointestinal tract. TSLP antibodies have the ability to specifically bind to human TSLP, blocking their interaction with the receptor complexes, effectively inhibiting the release of pro-inflammatory cytokines from TSLP-targeted immune cells, thereby preventing asthma exacerbation and improving asthma control. TSLP is a key mediator in various phenotypes of asthma, and antibody drugs targeting TSLP and its signal transduction are considered effective strategies for treating asthma. At present, most of the drugs targeting TSLP under clinical research are monoclonal antibodies, with the drug formulation being subcutaneous injection solutions, and the commercial competition is fierce. There is an urgent need to introduce differentiated inhaled products.
About LQ043H
LQ043H is an anti-TSLP VHH antibody drug with complete independent intellectual property rights developed by Novamab:
1. LQ043H is administered via inhalation, which allows the drug to directly reach the lung lesion area, acting quickly while avoiding the first-pass effect through the liver and reducing systemic exposure toxicity. It not only takes advantage of the benefits of inhalation administration but also avoids the side effects of subcutaneous injection;
2. LQ043H is suitable for commercially available portable nebulizers, greatly improving patient compliance;
3. LQ043H utilizes a Pichia pastoris production system, which is highly productive and has significant advantages for commercial promotion;
4. At present, the sequence, derivatives, and uses of LQ043H have applied for patent protection in countries/regions such as China, Europe, and the United States, and have obtained Chinese invention patent authorization (authorization announcement number CN114853888B).
5. The Phase Ia clinical study for the asthma indication of LQ043H in China has been completed, with good safety, and it is about to enter Phase Ib/II clinical studies.
About Novamab’s Pichia pastoris CMC platform
Novamab has successfully established a Pichia pastoris CMC (Chemistry, Manufacturing, and Control) platform and has accumulated extensive experience in the development of multiple complete projects. Over 90% of the projects on the Pichia pastoris CMC platform achieve a yield of more than 1g/L, and the purification platform process has an overall recovery rate of over 50%. The development time for projects has been significantly reduced, taking only 3-4 months from cell strain construction to protein purification. The small-scale and pilot-scale expression platforms established by Novamab include 12 units of 7L fermenters and 2 units of 100L fermenters, along with corresponding purification systems. In addition, a 500L Pichia pastoris pilot-scale GMP (Good Manufacturing Practice) certified cleanroom facility for clinical sample production has been stably utilized, enabling Novamab to have the capability to produce clinical Phase I and Phase II samples. The construction of the 500L GMP pilot production workshop for Pichia pastoris VHH antibodies has filled an industry gap in this field, offering a unique scarcity and competitive edge.



