 About us
About usNovamab is located in the Shanghai International Medical Park (Pudong New District). It is an innovative biopharmaceutical company in the clinical stage, dedicated to developing VHH antibody drugs targeting the multi-billion-dollar markets of respiratory diseases and inflammatory disorders. Our mission is to develop life-changing therapies for patients through the research and development of innovative nanobodies (VHHs), while also enhancing drug efficacy and usability.
The company utilizes the advanced NanoVibe™ VHH platform to fully leverage the advantages of nanobodies, creating highly differentiated antibody drug platforms, including the inhaled antibody platform (Aerobody), the oral antibody platform (Orabody), and the dual/multi-antibody platform (Pluribody). Our product pipeline includes the FIC Phase II clinical inhaled IL-4R nanobody, the Phase I clinical inhaled TSLP nanobody, and multiple TL1A dual/multi-antibodies and oral pipelines, targeting indications such as asthma, chronic obstructive pulmonary disease (COPD), and inflammatory bowel disease (IBD).
The company has attracted the attention and support of several well-known investment institutions and funds, including Zhang Ke LINGYI, Qiming Venture Partners, and Shengdi Investment, among others. We currently have approximately 60 full-time employees so far.
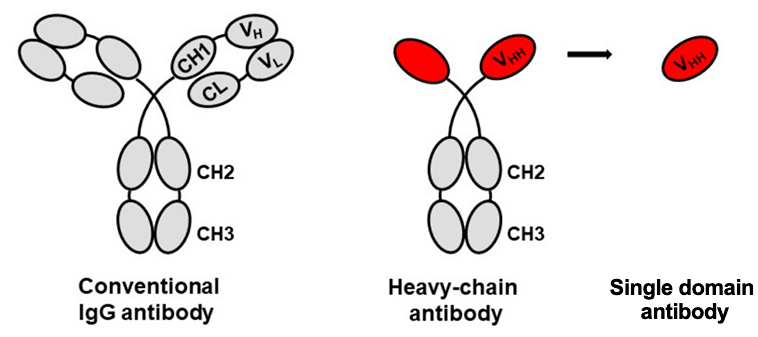
 Research and Development
Research and Development
Novamab currently has multiple VHH antibody new drug product chains, covering diseases such as respiratory diseases and autoimmune diseases, and has formed strategic partnerships with several well-known domestic and foreign enterprises.
 Common growth
Common growth