

Share the latest information



Recently, Novamab and the team of Professor Zhang Xuyao/Ju Dianwen from the School of Pharmacy, Fudan University, jointly reported that the VHH antibody-drug conjugate (NDC) molecule targeting TROP2 has played an outstanding anti-tumor role in the treatment of pancreatic cancer. The related results were published online in the Journal of Nanobiotechnology (with the latest impact factor of 10.2) under the title "TROP2-directed VHH antibody-drug conjugate elicited potent antitumor effect in pancreatic cancer." Xu Caili and Zhu Min are the co-first authors of this article, and Dr. Zhang Xuyao, Dr. Wan Yakun, and Dr. Ju Dianwen are the corresponding authors.
Pancreatic cancer, known as the "king of cancers," has a 5-year survival rate of only 12%. Its early symptoms are concealed, it is insensitive to immunotherapy, and the first-line therapy is mainly chemotherapy; however, it also faces problems of severe side effects and drug resistance, urgently requiring new treatment strategies and drugs. Trophoblast cell surface antigen 2 (TROP2) is overexpressed in the tumors of more than half of pancreatic cancer patients and has been identified as a potential target for antibody-drug conjugate (ADC) treatment of breast cancer. The currently reported TROP2-targeted ADC drugs are mainly IgG type and there is very little research on pancreatic cancer. VHH antibodies have advantages such as strong tissue penetration, high endocytosis efficiency, and low immunogenicity, and have great potential in the construction of ADCs.
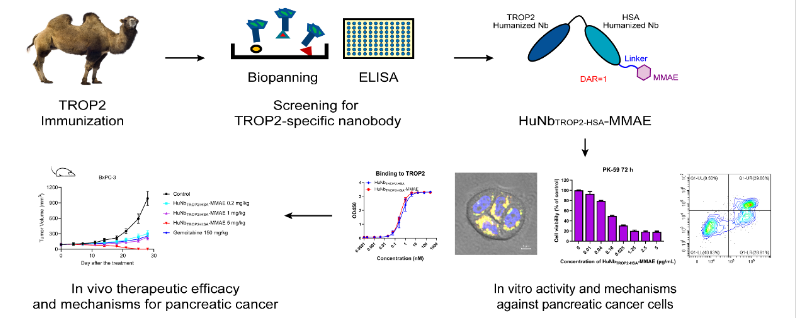
Novamab immunized camels with human TROP2 protein, constructed a phage display library, and obtained anti-TROP2 VHH antibody candidates through multiple rounds of screening. After humanization, they were conjugated with human serum albumin (HSA) and the auristatin-class toxin MMAE to develop a new type of TROP2-targeted NDC molecule HuNbTROP2-HSA-MMAE for the treatment of TROP2-positive pancreatic cancer. HuNbTROP2-HSA-MMAE binds specifically to TROP2 and is efficiently internalized by tumor cells within 5 hours, then transported to lysosomes and releases MMAE, inducing apoptosis of TROP2-positive pancreatic cancer cells through the caspase-3/9 pathway. The drug can maintain good plasma stability for several days, and can be quickly hydrolyzed by lysosomal proteases to release the small molecule toxin within hours. In the pancreatic cancer xenograft model, HuNbTROP2-HSA-MMAE has a high penetration rate into tumor tissues, can accumulate in tumor tissues for a long time, and induce tumor cell apoptosis. A dosing regimen of 5 mg/kg twice a week can even eliminate the xenograft. This research result provides new pathways and molecules for the treatment of pancreatic cancer.
About VHH antibody-conjugated drugs (NDC)
Currently, there are several approved ADC drugs for both hematological malignancies and solid tumors, but the development of ADC drugs in solid tumors is becoming more and more limited, mainly due to the large molecular weight of ADC drugs (IgG scaffold about 150 kDa), which results in poor tissue penetration in solid tumors. In contrast, NDC drugs based on nanobodies have significant advantages in tissue penetration. In addition, nanobodies are stable, easy to modify, and can form highly stable and uniform therapeutic drugs with good druggability.
About Novamab
Shanghai Novamab Biopharmaceuticals Co., Ltd. is an innovative biopharmaceutical enterprise dedicated to the research and industrialization of VHH antibody drugs. The company was established in October 2017 and is located in the Shanghai International Medical Park. It has established a comprehensive research and development, CMC, and pilot production platform and system, and is currently the only company in China with the ability to develop VHH antibodies throughout the entire process. Novamablogy has built a 500 L Pichia pastoris VHH antibody GMP pilot production workshop, filling the industry gap in this field, which is highly scarce and competitive.
As of October 2023, Novamab has applied for more than 80 domestic and international invention patents, with 26 authorized. Novamab has developed dozens of VHH antibody drugs, with a pipeline covering areas such as the respiratory system, anti-infection, ophthalmology, and oncology, and has now obtained four clinical trial approvals. As the lead inhalable products under investigation, LQ036 and LQ043, have revolutionized the treatment of respiratory diseases and are the world's first inhaled VHH antibody drugs targeting the same site. Phase I clinical trials for asthma indications for LQ036 has been conducted in China and Australia (completed), both showing excellent safety and tolerability. And Phase II prove of concept study for LQ036 would be conducted in Q1, 2024. The application for LQ036's COPD indication was approved by CDE in September this year. The Phase I clinical study for LQ043's asthma indication in China has also completed all dosing, demonstrating good safety. Phase II trial for LQ043 will be on its way soon. LQ043 could benefit non Th2-high asthma patients, and LQ043 and LQ036 could cover the entire moderate to severe asthma population.



