

Share the latest information




Novamab Announced Presentation of Encouraging Clinical Data for LQ036 at ATS 2023 International Conference
This year’s European Respiratory Society International Conference (ERS), the world's largest and most influential conference on respiratory diseases, was held in Milan, Italy on September 9-13. Novamab announced oral presentation of the clinical results for LQ036, which aroused great interests of several MNCs. LQ036 is the first inhalable IL-4Rα neutralizing single domain antibody independently developed by Novamab.
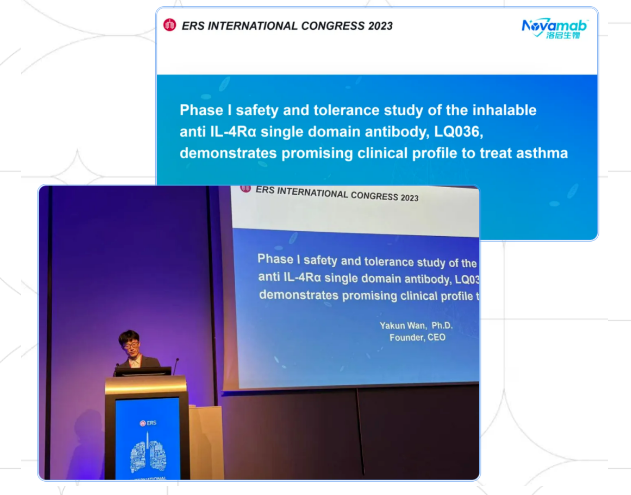
The Phase I clinical study data of LQ036 showed that both single and multiple doses of LQ036 were well tolerated in healthy subjects, and no drug induced immunogenicity caused was observed. There were no reports of serious or severe adverse events. The pharmacodynamic biomarker analysis in Phase I clinical studies in China preliminarily showed that LQ036 has excellent therapeutic potential. Recently, LQ036's new IND application for COPD has been approved by CDE, which is expected to bring significant clinical benefits to patients with type2 moderate to severe COPD.

About 2023 ERS
ERS is an international organization that brings together doctors, healthcare professionals, scientists, and other respiratory medical experts from over 160 countries worldwide. Its mission is to promote lung health, alleviate disease pain, and promote the development of respiratory medical standards worldwide. The ERS Conference is the world's largest academic conference on respiratory medicine, where professionals from various fields of respiratory medicine gather from around the world to showcase and discuss the latest
About LQ036
LQ036 showed excellent activity in preclinical studies, with a 5-fold and 10-fold increase in activity compared to Dupilumab and AZD1402/PRS060 respectively.
LQ036 is produced by Pichia pastoris, which could significantly shortens the production cycle compared to conventional monoclonal antibodies. And the high yieldcan reduce production costs by about 90%, greatly enhancing the competitiveness of the product. It has a huge competitive advantage in product commercialization promotion and drug penetration rate improvement.



