

Share the latest information



New · Medicine · Progress · Exhibition
On January 6, 2023, the China National Drug Administration Drug Evaluation Center (CDE) announced that the application for clinical trial (IND) of LQ043H single-domain antibody aerosol, a core drug for the treatment of moderate and severe asthma of Shanghai Luoqi Biomedical Technology Co., Ltd., was officially approved (Notice No.: 2023LP00043). This is also another nano-antibody drug for respiratory diseases that was approved in Phase I of China's clinical trial after LQ036 in 2022.
LQ043H aerosol, independently developed by Loki Biology, is an inhaled nanoantibody drug targeting TSLP, which is used to treat broad-spectrum moderate and severe asthma. Inhibition of human thymic stromal lymphopoietin (TSLP) has been proved to be an effective way to treat non-eosinophilic and eosinophilic asthma in recent clinical studies. TSLP is secreted by airway epithelial cells and is considered as "upstream" cytokine, which can activate DC and release chemokines, thus recruiting and activating Th2 cells; TSLP may also participate in airway wall remodeling by acting on lung fibroblasts.
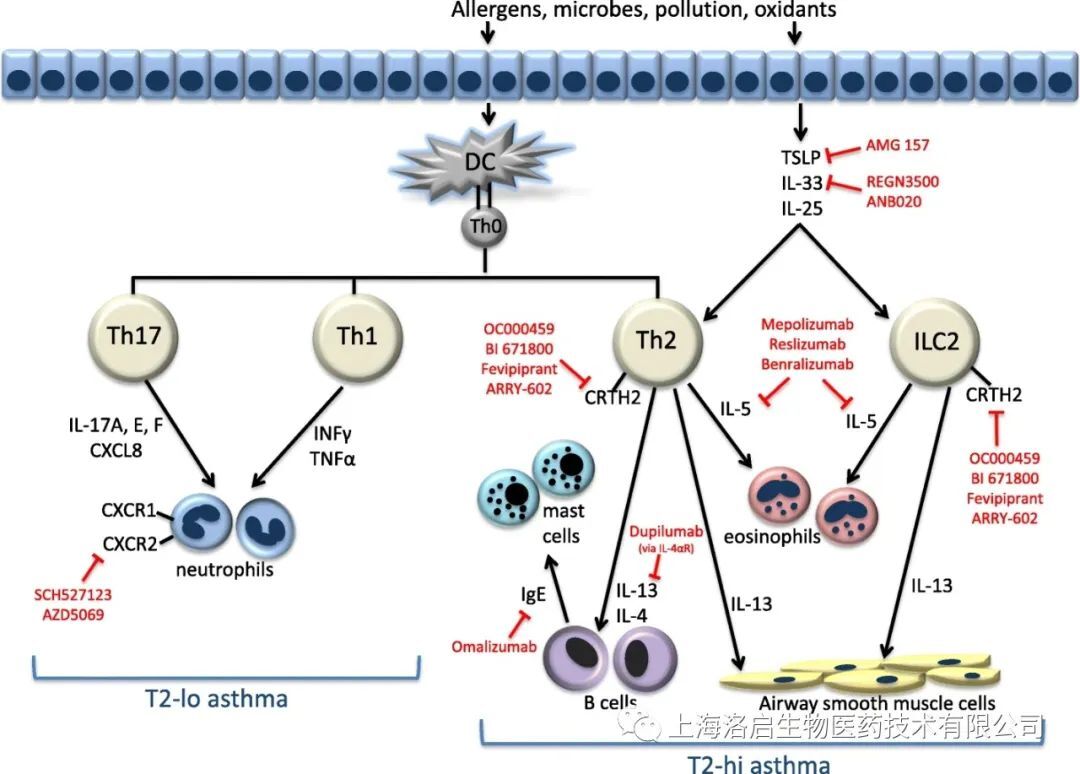
At present, most of the drugs in clinical research of TSLP target are monoclonal antibodies, and the drug dosage form is subcutaneous injection. The commercial competition is fierce, and the differentiated second-generation products need to be launched urgently. At present, in the research projects of global TSLP targets, three drugs are administered by inhalation, namely Ecleralimab (clinical phase II) of Novartis/Morphosys, AMG 104 (clinical phase I) of AZ/Amgen, and LQ043H (clinical phase I) of Loki Biological.
LQ043H has many product advantages and is expected to become the world's first nano-antibody aerosol that can treat broad-spectrum asthma, benefiting a wider range of asthma patients:
· Strong activity: This product has better activity advantages compared with the same target antibody drugs on the market.
· Good stability: This product has good storage stability and can be stored at room temperature for 2 years. This product can be used with a variety of portable atomizers. It has good quality stability and activity before and after atomization.
· Atomization administration directly reaches the focus, with small toxic and side effects: this product is atomized, and the drug is delivered directly to the lung, which takes effect quickly; And only a small amount of drugs enter the blood circulation system with low systemic toxicity.
· Pichia pastoris production with stable process and low cost: this product is obtained through Pichia pastoris expression and production with stable process; The expression yield is up to 20g/L, and the production cost is low, which lays a good foundation for its commercial promotion in the later stage.
About nano antibody drugs
At present, the nanoantibody industry is in the early stage of development and the market is active. Many large and medium-sized pharmaceutical enterprises are actively laying out nano-antibody-related pipelines. So far, four nanoantibody drugs have been approved for marketing:
1.Sanofi Cablivi (Caplacizumab), used for the treatment of adult acquired thrombotic thrombocytopenia;
2.Nvida of Corning Jerry ®, The world's first subcutaneous injection PD-L1 inhibitor;
3.The BCMA CAR-T product developed jointly by Janssen and Legendary Biology, Sidaki Orensai (trade name Carvykti), has been approved by the US FDA for marketing and is used to treat adult patients with recurrent/refractory multiple myeloma;
Ozooralizumab injection of Daisei Pharmaceutical Co., Ltd. was approved for marketing by Japan PMDA on September 26, 2022, and is applicable to rheumatoid arthritis. Oli Group single antigen research company is Ablynx, which is a human TNF α Nano antibody.
About Loqi Biological "inhaled macromolecular drug R&D platform”
Luoqi Biological has independently created five core technology platforms based on nano-antibody: nano-antibody rapid screening platform, Pichia pastoris CMC process development platform, inhaled macromolecular drug research and development platform, nano-antibody long-acting platform and nano-antibody dual-antibody platform, all of which have very unique industry advantages.
Among them, the research and development platform for inhaled macromolecular drugs of Luoqi Biological covers asthma, COPD, COVID-19, pulmonary fibrosis and other respiratory diseases, including IL-4R, TSLP, COVID-19 S protein and other popular targets; The drug directly reaches the focus, with high utilization rate and low toxicity and side effects; Good portability with small atomizer, stable quality before and after atomization. At present, LQ036, the first drug of inhaled macromolecular drug R&D platform, is carrying out phase I clinical research in Australia and China, showing good safety and tolerance.



