

Share the latest information



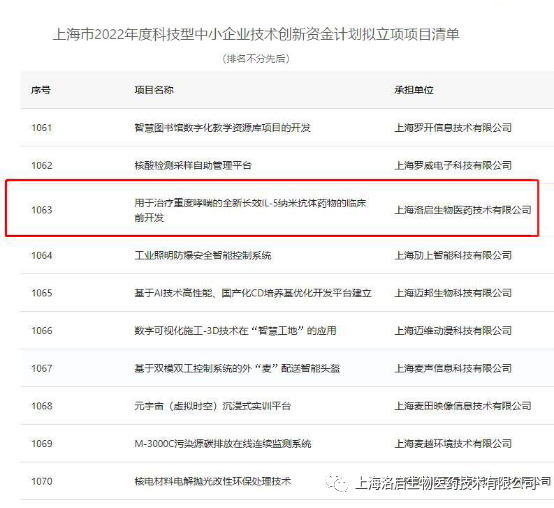
Recently, the list of projects to be approved for the "Shanghai 2022 Technology Innovation Fund Plan for Small and Medium-sized Scientific and Technological Enterprises" organized by the Shanghai Municipal Commission of Science and Technology has been publicized, and the project of "preclinical development of a new long-acting IL-5 nanoantibody drug for the treatment of severe asthma" of Loqi Biological has been shortlisted.
Shanghai 2022 Technological Innovation Fund Plan for Science and Technology SMEs
The 2022 "Technological Innovation Fund Plan for Small and Medium-sized Technological Enterprises" is selected and publicized by the Shanghai Municipal Commission of Science and Technology in accordance with the unified deployment of the Municipal Party Committee and the Municipal Government, by organizing experts in a unified way, by means of competitive roadshows and online reviews. Innovation funds mainly support enterprises with innovative capabilities and high growth potential, and focus on supporting innovation and entrepreneurship projects in high-tech fields, such as the transformation of scientific and technological achievements with independent intellectual property rights.
The targeted IL-5 long-acting nanoantibody developed in this project has a very significant eosinophil inhibition effect, which is 100 times the activity of the three commercially available drugs with the same target, and its drug delivery cycle can be up to three months. Compared with the existing products, which are administered once a month, it has better compliance and market prospects. The completion and improvement of this project is expected to become the world's first fully independently developed and industrialized long-acting IL-5 nanoantibody innovative drug, and become a blockbuster product of the new generation of moderate and severe asthma drugs, making up for the deficiency of this targeted monoclonal antibody drug in clinical research and development.
About novamab
Shanghai Luoqi Bio-pharmaceutical Technology Co., Ltd., located in Shanghai International Medical Park, Pudong New Area, Shanghai, is a clinical stage company dedicated to the research and development of innovative drugs for nano-antibody. Luoqi Bio-pharmaceutical has a high-level foundation for the research and development of nano-antibody drugs, has established an integrated, systematic and comprehensive professional development platform, and is the only company in China with the ability to develop nano-antibody drugs in the whole process. Loki Biological has built a 500L Pichia pastoris nanoantibody GMP pilot production workshop, filling the industry gap in this field, which is extremely scarce and competitive.
At present, Rocky Biology has many new drug product chains of nanoantibodies, covering respiratory diseases, autoimmune diseases, tumors and other fields. The unique innovative inhalation macromolecular drug research and development platform of LUKIBIO covers asthma, COPD, COVID-19 and other respiratory diseases; The drug directly reaches the focus, with high utilization rate and low toxicity and side effects; Good portability with small atomizer. The first product of the platform, LQ036, the world's first inhaled macromolecular asthma treatment drug, entered phase I clinical trials in Australia in June 2021, and human experiments showed excellent safety; In January 2022, the Chinese clinical phase I IND of the project has also been officially approved, and the Chinese clinical phase I will be carried out simultaneously. In addition, LQ043, the second product of the platform, has also been submitted to the domestic Pre-IND.



