

Share the latest information



Recently, Novamab, with its leading advantages in the field of nano-antibody innovative drugs, as well as its achievements in the construction of intellectual property system and the promotion of clinical pipelines, has been listed in the "Top 100 Chinese pharmaceutical innovative seed enterprises". The list was selected by the world's leading life science professional information service provider and database "Clarivate" and the well-known medical media "E-drug manager" after four months of data screening, integration and analysis.
Top 100 Chinese pharmaceutical innovation seed enterprises
The "Top 100 Seeds" is based on the evaluation system of innovation foundation, innovation process and innovation achievements, and is based on Korui Weian Derwent ™ Patent data and Cortellis ™ Competitive intelligence and clinical trial data are selected from more than 5000 Chinese pharmaceutical enterprises through the data processing of the number of authorized patents, the total number of patent citations, the number of clinical trials under study, and the number of approved and marketed new drugs. The listed enterprises have great potential in licensing patents, patent citation, clinical trials and other aspects. As the first camp representing China's pharmaceutical innovation potential, the "Top 100 Seed" enterprises are an important backup force for the transformation and upgrading of China's pharmaceutical industry and the building of industrial competitiveness. They provide a model for seed enterprises to grow in the positive direction while tapping the innovation potential of the industry.
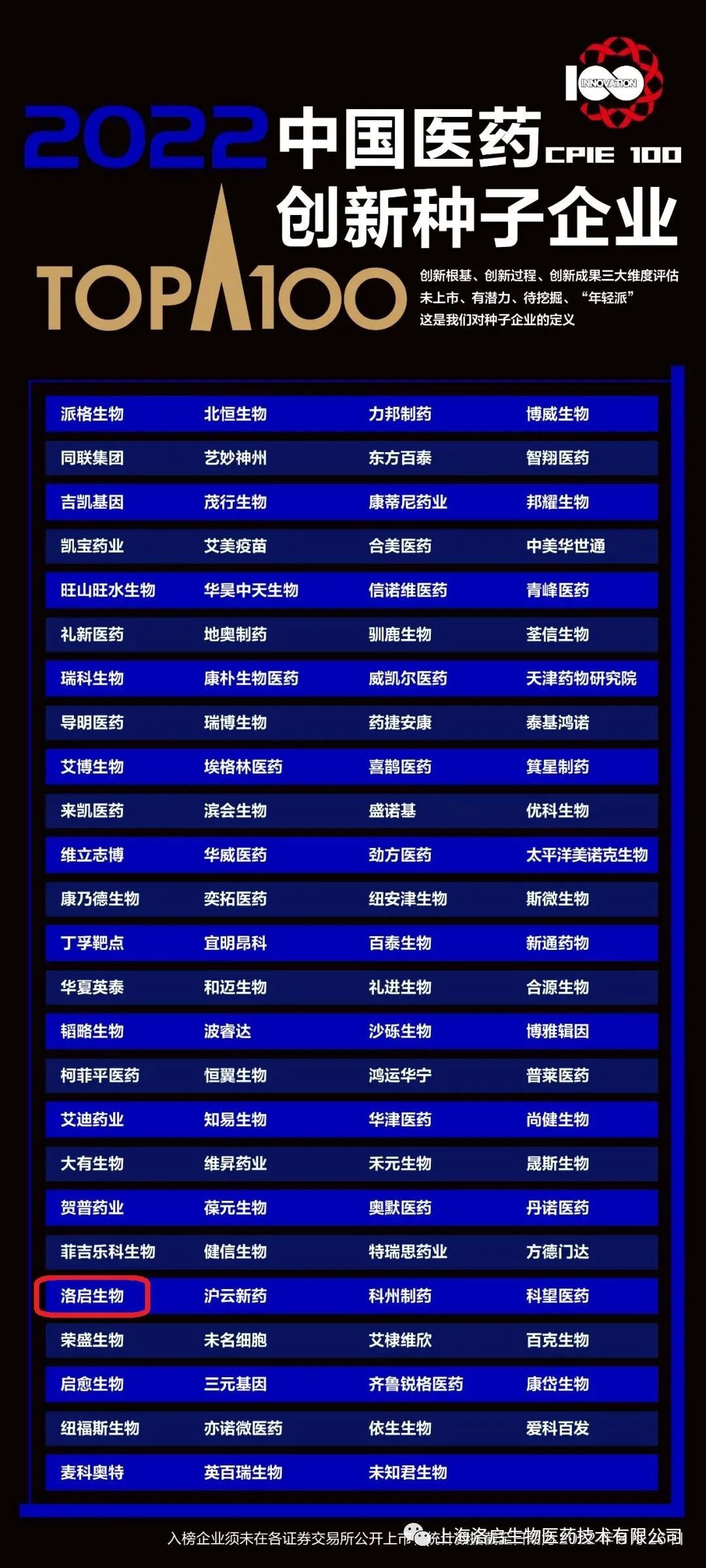
The inclusion of Rocky Biology in the list is a high affirmation of its R&D innovation and comprehensive competitiveness. In the future, Loqi Biology will adhere to the concept of "providing patients with newer and safer innovative drugs", continue to increase investment in high-tech research and development, continuously improve its independent innovation ability, and strive to realize the new drug dream of "Camel Antibody Starts a Healthy Future" as soon as possible.
About novamab
Novamab, located in Shanghai International Medical Park, Pudong New Area, Shanghai, is a clinical stage company dedicated to the research and development of innovative drugs for nano-antibody. Luoqi Bio-pharmaceutical has a high-level foundation for the research and development of nano-antibody drugs, has established an integrated, systematic and comprehensive professional development platform, and is the only company in China with the ability to develop nano-antibody drugs in the whole process. Loki Biological has built a 500L Pichia pastoris nanoantibody GMP pilot production workshop, filling the industry gap in this field, which is extremely scarce and competitive.
At present, Novamab has many new drug product chains of nanoantibodies, covering respiratory diseases, autoimmune diseases, tumors and other fields. The unique innovative inhalation macromolecular drug research and development platform of Novamab covers asthma, COPD, COVID-19 and other respiratory diseases; The drug directly reaches the focus, with high utilization rate and low toxicity and side effects; Good portability with small atomizer. The first product of the platform, LQ036, the world's first inhaled macromolecular asthma treatment drug, entered phase I clinical trials in Australia in June 2021, and human experiments showed excellent safety; In January 2022, the Chinese clinical phase I IND of the project has also been officially approved, and the Chinese clinical phase I will be carried out simultaneously. In addition, LQ043, the second product of the platform, has also been submitted to the domestic Pre-IND.



