

Share the latest information



It’s very comforting to see the acceptance of VHH antibody drugs in the field of biopharmaceuticals for a biotech focusing on innovative VHH antibody drugs, such as Novamab. Nanoantibodies have the advantages of simple humanization, high affinity, good stability, low immunogenicity, great permeability, and noble solubility. VHH antibodys have great potential in the future and are expected to play a role in innovative therapies and disease diagnosis. VHH antibodys are gradually moving from scientific research to industrialization.
The following article is sourced from the Medicine Hunter Club, written by the Medicine Hunter Club;
With the launch of the world's first VHH antibody drug Caplacizumab (Cablivi), the curtain of Nanobodies entering the market has officially opened. According to Sanofi's 2022 annual report, Cablivi's annual sales reached 211 million euros, almost quadrupling its sales in its first year of approval. Even more, the global sales of Cablivi was reported 113 million euros in 2023H1. The market performance of another VHH antibody drug Ozoralizumab, which was just launched in the second half of 2022, is also highly anticipated.
As the first VHH antibody drug launched in China, Envolizumab has only been on the market for over a year and achieved sales of over 560 million in 2022. In 2023H1, the sales revenue of the drug exceeded 350 million, and the R&D company Alphamab received 117 million yuan in revenue as a result. The other two partners, 3D Medicines and Simcere have also benefited greatly from this drug. This achievement is still commendable for the newly launched and novel subcutaneous administration of PD-L1 VHH antibody drug, with rapid volume expansion and promising sales growth.
Within 5 years since the first VHH antibody drug launched, various players in the VHH antibody drugs filed have emerged and showcased their skills in the market.

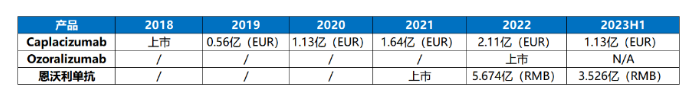
Sales of globally marketed VHH antibody drugs in H1 2023
2018 was the first year for VHH antibody drugs to enter the market, with a total of three VHH antibody drugs (excluding cell therapy drugs) being launched in the past five years. The world's first VHH antibody drug Caplacizumab achieved sales of 113 million euros in the first half of 2023, while the first VHH antibody drug in China also achieved sales of 352.6 million yuan in the first half of 2023. Both drugs are expected to hit new sales highs by the end of the year.
Ozoralizumab, which was just approved in Japan in September 2022, is currently on the market for less than a year. However, its unique dual specific VHH antibody design and significant therapeutic effects have also raised market expectations for it.
1、 Analysis of VHH antibody Drugs Launched Abroad
Currently, two VHH antibody drugs (excluding cell therapy products) approved abroad are all from Ablynx. This enterprise, founded in 2001, has been deeply involved in the field of Nanobodies for more than 20 years, and finally bloomed and fruited after years of research and development. With the acquisition of Ablynx by Sanofi, the foreign VHH antibody industry has officially welcomed the entry of leading MNCs. The two VHH antibody drugs, which were launched in 2018 and 2022 respectively, have done a lot of enlightening work for the development of the industry and are worth being taken as an example for in-depth consideration.
1.Global First - Ablynx: Caplacizumab

As the world's first approved VHH antibody drug, the significance of Caplacizumab milestone goes without saying. The release of this drug has also placed its developer Ablynx at the forefront of the global VHH antibody industry. In September 2018, Caplacizumab (trade name: Cablivi ®) was approved by EMA for the treatment of a rare blood disease - adult acquired thrombocytopenic purpura (aTTP). In February 2019, FDA also approved Cablivi ® combined with plasma exchange and immunosuppressive therapy for aTTP, which is also the first FDA approved therapy specifically for the treatment of aTTP. Since its approval, Caplacizumab's salesincreased year by year, with sales of 56 million euros in its first year of approval. According to Sanofi's annual report, sales have risen to 211 million euros by 2022, with 113 million euros in revenue in the first half of this year. The market outlook is very promising.
As the first VHH antibody drug launched, Caplacizumab has many areas worth learning from in its subsequent research and development. And the molecular design of Cablivi fully leverages the advantages of VHH antibody. In terms of configuration, it is a humanized bivalent VHH antibody comprised of two vascular hemophilia factor (vWF)targeting Nanobodies with a flexible linker, taking advantage of the small size and easy modification of Nanobodies, while increasing the number of contacts with the target.
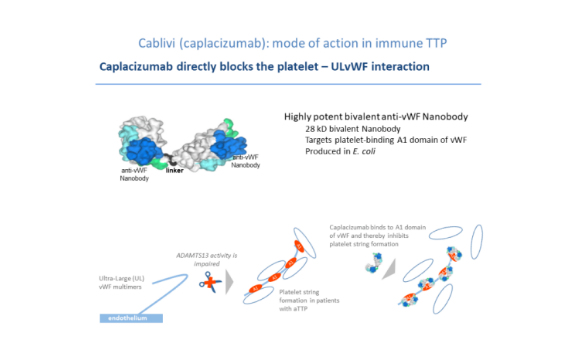
From the perspective of indications, Caplacizumab avoided popular targets and indications, and chose a rare blood disease aTTP in a new way. The occurrence of this disease is due to the fact that the protein vWF involved in hemostasis in the patient's body cannot be cleaved due to the impaired activity of the enzyme ADAMTS13, leading to the formation of a more active ultra large polymer UL-vWF and mediating platelet aggregation. The current treatment for this disease is not effective. Caplacizumab targets the A1 domain of vWF to prevent UL-vWF from adsorbing platelets to achieve therapeutic effects. This method not only has good therapeutic effect, but also the VHH antibody adsorbed on the high molecular weight UL-vWF actually delay the time of being cleared by the body and increase the half-life. The clever design strategy of leveraging strengths and avoiding weaknesses, as well as the unique selection of rare disease tracks, are the key to Caplacizumab's success and are widely discussed.
2.The first bispecific VHH antibody - Ablynx: Ozoralizumab

After the successful launch of Caplacizumab, another VHH antibody drug Ozoralizumab also derived from Ablynx. Ozoralizumab is also a landmark drug as it is the world's first bispecific VHH antibody drug approved for marketing, targeting TNF- α And HSA. Ozoralizumab was originally developed by Ablynx.. In September 2014, Ablynx authorized the development and commercialization of Ozoralizumab in Greater China to Eddingpharm. In June 2015, Ablynx licensed the development and commercialization of Ozoralizumab in Japan to Taisho Pharmaceutical. On September 26, 2022, Ozoralizumab developed by Ablynx was approved for launch in Japan (trade name: Nanozora ®)for the treatment of rheumatoid arthritis (RA), especially suitable for patients who have insufficient response to existing treatments.
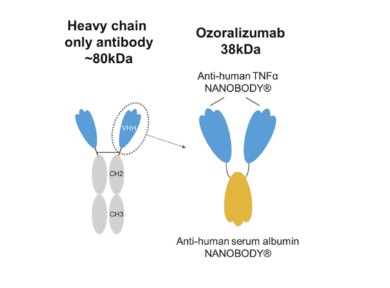
Ozoralizumab is a trivalent bispecific antibody in terms of configuration. It contains three humanized VHH antibody domains, with two of which target human TNF- α, another targeting HSA. It is worth mentioning that as a bispecific VHH antibody, Ozoralizumab uses TNFα part to take effect,and uses HSA part to extend the half-life. This approach also effectively compensates for the short half-life of Nanobodies due to their small size, resulting in an average half-life of 18.2 days after a single administration. This VHH antibody targeting HSA as an auxiliary group has also become a commonly used method for extending the half-life of drugs, applied in many companies' drugs.
Ozoralizumab has significant therapeutic effects on RA. The Phase II/III clinical trial "OHZORA" demonstrated that the ACR20 response rate of the Ozoralizumab treatment group at week 16 was 79.6%, significantly higher than the 37.3% response rate of placebo. The Phase III clinical trial "NATSUZORA" demonstrated that the ACR20 response rate of the Ozoralizumab treatment group at week 24 was 82.5%, significantly higher than the 57.8% of the methotrexate (MTX) treatment group. Due to less than a year of launch, there is currently limited sales data information. It is believed that excellent therapeutic effects will lead to good sales performance and market opportunities for Ozoralizumab in more countries in the future.
2、 Introduction to domestic launched VHH antibody drugs
Although domestic enterprises did not start in the field of Nanobodies as early as abroad, their steady and rapid research and development speed has enabled the domestic VHH antibody industry to keep up with the progress of foreign countries. In recent years, many high-quality drugs or pipelines have also emerged. At present, a VHH antibody drug called Envolizumab was launched earlier than Ozoralizumab, and has also achieved good market results.
The first domestic VHH antibody drug - Alphamab: Envolizumab

If the launch of Caplacizumab is a milestone in the global VHH antibody market, then the launch of Envolizumab developed by Alphamab is a milestone in the domestic VHH antibody market. In November 2021, Nvida ® (Envolizumab Injection) was approved for marketing in China and is suitable for the treatment of adult advanced solid tumor patients with unresectable or metastatic microsatellite highly unstable (MSI-H) or mismatch repair gene deficient (dMMR).
Although Envolizumab was developed by Alphamab, a tripartite partnership was established in the later stage, consisting of Alphamab, 3D Medicines, and Simcere. In February 2016, while Envolizumab was still in the research and development stage, 3D Medicines reached an agreement with Alphamab, with Alphamab and 3D Medicines each accounting for 49% and 51% of the commercialized rights. On March 30, 2020,Simcere also joined the cooperation. Finally, Alphamab is responsible for production and supply, 3D Medicines is responsible for clinical development, registration and sales, and Simcere is responsible for exclusive commercial promotion after launch in Chinese Mainland.
The successful launch of Envolizumab did not disappoint the expectations of the three partners. Throughout 2022, Envolizumab achieved sales of over 560 million. Alphamab earned 148 million yuan in revenue from this drug, an increase of 1171.1% compared to the newly launched year 2021. As the only launched drug in the company, it dominates the total revenue of 167 million yuan for the entire year. As a salesperson,3D Medicines recorded a revenue of 567.4 million yuan, and as the only drug launched on 3D Medicines market, Envolizumab also supported 3D Medicines annual revenue performance, becoming 3D Medicines "storefront responsibility". For Simcere, the rapid release of Envolizumab has also driven the revenue growth of the company's tumor sector, becoming one of the "3+1" growth engines of Simcere.
After entering 2023, Envolizumab still maintains a strong momentum. According to the positive profit forecast for 2023H1, in the first half of 2023, the revenue of Envolizumab included in the plan has reached 352.6 million, an increase of 70.3% year-on-year. The newly released 2023 semi annual report of Alphamab revealed that Envolizumab brought revenue of 117 million to Corning Jerry in the past six months, an increase of 118.87% year-on-year. According to the 2023 semi annual report of Simcere, the rapidly growing Envolizumab and another tumor drug of the company, Envolizumab, jointly drove a year-on-year growth of 34.8% in the tumor sector in the first half of the year. Overall, the Envolizumab has achieved impressive results and is expected to continue to benefit the three partners in the long term in the future.
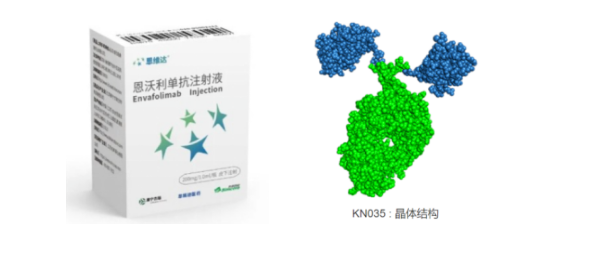
Looking back at the development process of this drug, Alphamab used PD-L1-Fc fusion protein to immunize Xinjiang Bactrian camels four times, and finally screened the most affinity VHH antibody using a phage display library established from peripheral blood. The VHH antibody was humanized and fused with the Fc domain to increase the half-life of the VHH antibody, ultimately obtaining Envolizumab.
The results of the Phase II clinical study in China showed that the objective response rate (ORR) of Envolizumab monotherapy (administered subcutaneously with 150 mg QW) for 103 MSI-H/dMMR solid tumor patients was 44.7%, including 65 colorectal cancer patients, with an ORR of 43.1%. The median progression free survival (PFS) of the total population was 11.1 months, and the 1-year overall survival (OS) rate was 73.6%. This positive result has produced a good transcript for the development of Envolizumab.
It is worth mentioning that Envolizumab is the world's first and currently the only approved subcutaneous injection of PD - (L) 1 antibody. It is relatively stable at room temperature, with fewer restrictions on the injection site, and can be easily administered, shortening the administration time and having lower medical costs. The ability to achieve this characteristic is inseparable from the high thermal stability of VHH antibody. At the same time, the success of the PD - (L) 1 target in antibody development also provides a guarantee for the development of VHH antibody. But as a popular target, fierce competition is also inevitable. By cleverly leveraging the advantages of VHH antibody and choosing subcutaneous injection as the drug delivery method, Envolizumab can also stand out and shine brightly.
Alphamab is moving forward in the development of Envolizumab and actively expanding its application prospects. At present, multiple indication pipelines of Envolizumab are steadily advanced. Recently, the combination of enrolizumab and SOX regimen (oxaliplatin+tiggio) for the treatment of metastatic/recurrent gastric adenocarcinoma has been published in ASCO. The results showed an ORR of 50% and a DCR of 87.5% in 38 patients, indicating that the combination of enrolizumab and SOX regimen is a promising treatment option for metastatic/recurrent gastric adenocarcinoma.
In addition, the phase 2 clinical results of the combination of Envolizumab and Cedarbenzamide in the treatment of functional cure of HIV infection have recently been released, indicating that the therapy has good tolerance and effectively activates the latent HIV virus library. The cell-related HIV RNA levels of the subjects at week 8 and week 12 significantly increased compared to baseline levels, with an average increase of 4.27-fold and 3.41-fold, respectively. The ratio of HIV cell related RNA to total DNA also shows the same trend. The optimistic results also bring hope for the antiviral field of Envolizumab beyond anti-tumor therapy.
VHH antibody drugs are beginning to emerge in the domestic market. And with the steady development of the international industry, the VHH antibody drug race is poised to take off. While commenting on the pioneers of global VHH antibody drugs on the market, we also set our sights back to China and selected some of the unique research related pipelines to see how the future "trendsetters" in these tracks will perform.

Potential Domestic VHH antibody Drugs
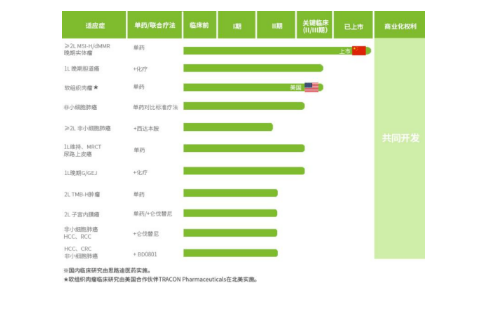
For the domestic VHH antibody industry, in addition to a launched drug, there are also many representative VHH antibody related pipelines, which have their own characteristics in configuration, dosage form, modification, and use based on the characteristics and properties of Nanobodies themselves. We will introduce these representative drug pipelines and glimpse the diversified development trend of the domestic VHH antibody industry.
1.Bispecific VHH antibody - Alphamab: KN046

After the success of Envolizumab, Alphamab leveraged its advantages in the field of bispecific antibodies and pushed forward the innovative bispecific VHH antibody pipeline KN046. This is a bispecific VHH antibody that targets both PD-1 and CTLA-4 simultaneously. Two Nanobodies targeting different targets are concatenated on a single chain of the antibody and fused with the Fc domain. This antibody targets two types of immune checkpoints and can target tumor microenvironments enriched in PD-L1 high expression and clear Treg, which inhibits tumor immunity.
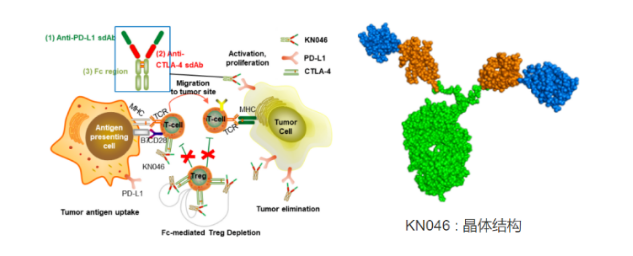
KN046 has carried out nearly 20 clinical trials in different stages covering more than 10 kinds of tumors in Australia, the United States and China, including non-small cell lung cancer, pancreatic cancer, thymus cancer, liver cancer, esophageal squamous cell cancer, and triple negative breast cancer. Among them, the clinical treatment of squamous NSCLC and pancreatic cancer combined with chemotherapy has progressed to phase III. Previously, Alphamab released phase II clinical data on KN046 for the treatment of advanced NSCLC. The results showed that KN046 was well tolerated and effective as a second-line treatment for advanced NSCLC. It showed benefits in progression free survival (PFS) and overall survival (OS) in non-small cell lung cancer. The median follow-up period was 13 months, and the median progression free survival period was 3.68 months (95% CI 3.35, 7.29). The squamous and non squamous NSCLC were 7.29 months (3.68, 9.23) and 3.58 months (2.46, 5.52), respectively; The 6-month survival rate is 85.6%, and the 12-month survival rate is 69.7%. Compared with the historical data of PD (L) -1 antibody, it shows advantages.
The dual target selection of KN046 is also highly competitive, for companies such as Akesobio and Baili-pharm also have the same target layout. It remains to be further observed whether KN046, as a unique design strategy for VHH antibody, can continue to show advantages. However, the future progress of KN046 may not always be smooth sailing. Recently, Alphamab announced the unblinding of phase 3 trial KN046-301 was not successfully completed due to lack of statistically significant differences in OS, and the trial is still ongoing. Although it did not fail, it still cast a layer of uncertainty over the drug. Compared with NSCLC, KN046 deserves more attention in the clinical treatment of pancreatic cancer. Since there is no PD-1 antibody effective against pancreatic cancer at present, if KN046 can succeed, it will undoubtedly bring important benefits to Alphamab. Just like the continuous launch of two VHH antibody drugs by Ablynx, Alphamab also has the expectation of replicating this achievement in China. Therefore, KN046 has an undeniable influence on Alphamab itself and the domestic VHH antibody race.
2.Inhalation administration - Novamab: LQ036

Being able to tolerate nebulized administration is a major advantage of VHH antibody. As a company deeply rooted in the vertical field of Nanobodies, Novamab also attaches great importance to the use of inhalation drug delivery to develop products. According to the information provided on the official website of Novamab, several of the company's research and development pipelines are inhalation based, mainly targeting respiratory diseases. For this type of disease, inhalation administration often brings more direct therapeutic effects.
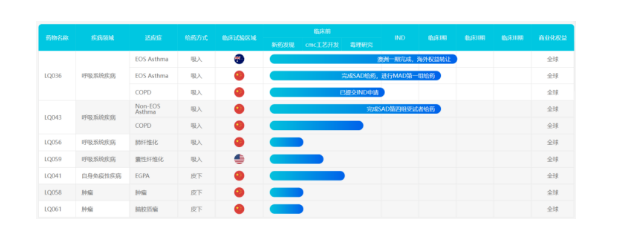
LQ036 is one of the fastest progressing research and development pipelines in Novamab, targeting indications for moderate to severe asthma. Currently, it has completed Phase I clinical trials in Australia. LQ036 is a VHH antibody against IL-4R, targeting the IL-4/IL-4R pathway to treat severe asthma. Asthma is a major persistent disease in the medical community, with approximately 350 million people worldwide suffering from it. Asthma is closely related to Th2 cell-mediated type 2 inflammatory response, and IL-4 can promote the differentiation of CD4+T cells into Th2 cells to promote type 2 inflammatory response. Therefore, blocking the IL-4/IL-4R pathway is an effective strategy for treating asthma. Currently, the only biologic drug targeting IL-4/IL-4R is Sanofi/Regeneron’s Dupixent, with sales reaching $6.198 billion in 2021, making it a very large market. However, the drug is a traditional monoclonal antibody injected subcutaneously, administered at a dose of 300 milligrams.
And LQ036 of Novamab was delivered to the pathological area of the lung, which has the advantages of fast onset of action, and lower systemic toxicity. It not only takes into account the advantages of inhalation administration, but also avoids the side effects of subcutaneous administration. LQ036 also takes advantage of the low cost of VHH antibody that can be expressed in Pichia pastoris. According to Novamab, the activity of LQ036 is 10 to 30 times that of Dupixent, and the process cost is only one ninth that of Dupixent. In addition to asthma, LQ036 has also been approved clinically for indications for chronic obstructive pulmonary disease.
In addition to LQ036, another drug, LQ043, is also undergoing Phase I clinical trials. This drug targets TSLP and can also effectively inhibit the inflammatory response. LQ043 is expected to become the world's first inhaled VHH antibody drug that can treat broad-spectrum asthma, benefiting a wider range of asthma patients.
3.Nuclide Coupled VHH antibody Diagnostic Agent - SmartNucl: SNA002

Nanobodies are also an excellent choice as diagnostic agents in addition to being therapeutic drugs. Traditional monoclonal antibodies have poor tissue penetration and long retention time in the blood, which is not conducive to their application in specific location enrichment diagnosis. It has been reported that traditional antibody diagnostic agents labeled with nucleotides require 6 days to obtain optimal tissue imaging. In contrast, Nanobodies have good tissue penetration, fast blood clearance, and can reach tumor tissue for optimal imaging in just a few minutes. In addition, Nanobodies have a short clearance time in the body and will not allow harmful radioactive substances to remain in the human body for too long, which is very suitable for the needs of instant diagnosis. They are designed as molecular diagnostic imaging probes.
Similar to the currently popular traditional antibody conjugated drug ADC, Nanobodiescan also be modified by coupled entity. Nanoantibodies coupled with radioactive nuclides are a typical representative, which can be used as both therapeutic drugs and diagnostic molecular probes. When used as a molecular probe, due to its short imaging time, it is more advantageous to couple some short half-life radioactive nuclides, such as 68Ga (t1/2=68 min), 18F (t1/2=110 min), or 64Cu (t1/2=12.7 h).
SSmartNucl, which deeply cultivates innovative nuclear drugs, has multiple nuclide coupled VHH antibody pipelines. Among them, SNA002, which has made the fastest progress, obtained IND approval from the US FDA in April and June 2022, as well as IND approval from China's CDE, and is about to enter clinical trials. This marks SNA002 becoming the first radioactive nuclide developer drug to receive dual IND approval from the US and China.

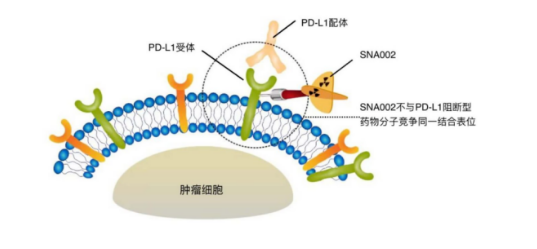
SNA002 is a VHH antibody radiotracer targeting PD-L1 developed based on the SRC platform of SmartNucl. It is labeled with 68Ga of radiation and can be visualized through PET-CT 1-2 hours after injection. It dynamically displays the expression levels of PD-L1 in various organs of the body in real-time, including heterogeneous expression within tumors and small metastatic lesions, enabling precise screening of effective populations for PD-1 treatment and guiding precise medication. It also avoids traditional invasive sampling methods, reduces patient pain, and allows for imaging examinations at any time based on the development of the disease. SNA002 suggests another use of antibodies targeting PD-L1 in addition to treatment, as a detection probe to assist in the use of other PD - (L) 1 therapeutic drugs. In addition, according to the official introduction of SmartNucl, the VHH antibody epitope of SNA002 is not the same as other therapeutic PD-L1 antibodies, and will not cause competitive binding or drug conflicts during use.
In addition to SNA002, SmartNucl also has another VHH antibody coupled nuclide diagnostic agent SNA006 that targets CD8 and has been declared IND. This diagnostic agent can detect TIL in tumors and provide a reference for immunotherapy.
4.Challenge the blood-brain barrier - LINNO PHARMA: LIN-2001, LIN-2008

The good tissue penetration of VHH antibody is also reflected in their advantages in penetrating the blood-brain barrier, which makes them more effective in dealing with some central nervous system diseases compared to traditional antibodies.
LINNO PHARMA is a pharmaceutical company that focuses on complement and macromolecular brain penetration technology. The blood-brain barrier drug delivery platform developed by LINNO PHARMA effectively utilizes the normal physiological and biochemical pathways of metabolism in the body, effectively avoiding potential neurotoxicity, maintaining a relatively high efficiency of drug delivery across the blood-brain barrier, and facilitating drug molecular structure modification and production. By utilizing this technological platform, LINNO PHARMA is driving a series of central nervous system diseases forward.
According to the data retrieval of the "single domain antibody" drugs by the Medical Cube, LINNO PHARMA's two drugs, LIN-2001 and LIN-2008, respectively, involve neurological diseases, both of which are VHH antibody drugs. LIN-2001 is a VHH antibody targeting complement, with indications for ischemic stroke and neurodegenerative diseases; LIN-2008 is a VHH antibody targeting VEGF, indicated for glioblastoma. Both drugs are in the preclinical development stage.
It is obvious that LINNO PHARMA should use its macromolecular brain penetrating technology to assist drugs in crossing the blood-brain barrier for both drugs used to treat brain tonic diseases. However, the form of VHH antibody itself provides convenience for brain penetration, and the combination of the two will undoubtedly increase the drug's brain penetration efficiency. This may also be the reason why LINNO PHARMA chose and developed this form of VHH antibody.

Summary
The time for VHH antibody to enter the market is not long, and the number of listed players on the track is not high. The selection of indications and research and development design are different from each other. Overall, the performance of these marketed drugs in the market is commendable. However, given the similarities and differences between VHH antibody and traditional antibody, the main competitors of VHH antibody drugs still come from traditional antibody drugs. In the future, more scenarios for the development of VHH antibody drugs will be placed under the competition of antibody drugs. It can be imagined that more VHH antibody products will follow the footsteps of the "pioneers" and enter the market in the future. In the face of fierce market competition, it is necessary to fully utilize the characteristics of VHH antibody, identify the positioning of their respective products, and fully absorb the research and development experience of successfully marketed drugs in order to stay ahead of others in the competition. However, we are also pleased to see that many distinctive pipelines have emerged in China's VHH antibody race track, and we believe that in the future, these pipelines can also break out of their own differentiated competitive routes.