

Share the latest information



Recently, the long-acting IL5 nanoantibody developed by Loki Biology can be used to treat eosinophil-related diseases, including eosinophilic asthma (EOS-Asthma), hypereosinophilic syndrome (HES) and eosinophilic granulomatous vasculitis (EGPA). The relevant research results were published in the journal "Research Research", entitled "Preclinical development of a long-acting ternary biological VHH antibody targeting IL-5 for the treatment of eosinophilic astroma" (IF=7.162). The corresponding author of this article is Dr. Wan Yakun of Shanghai Novamab.
Asthma is a chronic airway inflammatory disease characterized by airway hypersensitivity, reversible airflow obstruction, bronchial smooth muscle spasm and airway inflammation. There are 350 million people worldwide suffering from asthma, which is the second leading cause of death and disability after cancer. According to statistics, 80% of asthma patients are eosinophilic asthma. IL-5 is involved in various mechanisms of eosinophils from differentiation, development, proliferation, survival to anti-apoptosis, so it targets IL-5 or its specific receptor IL-5R α, It has become one of the main strategies for treating severe asthma.
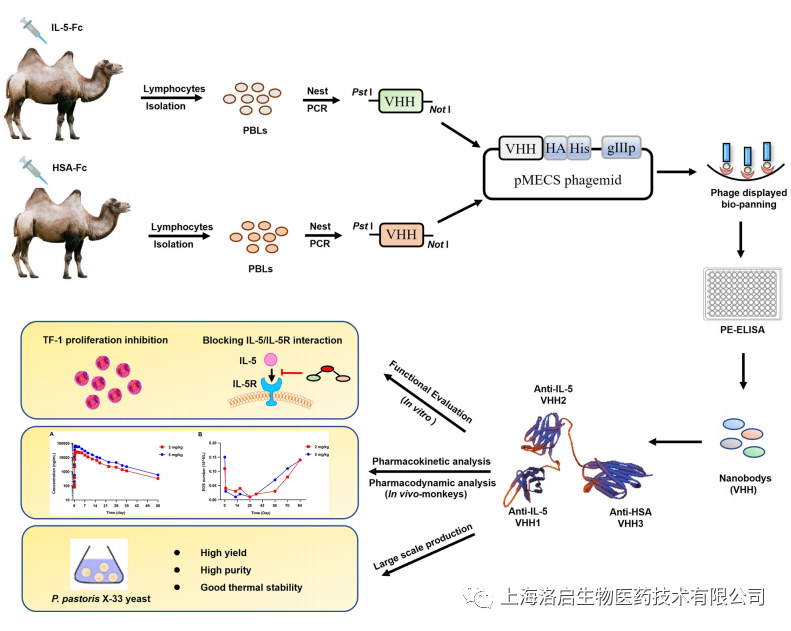
In this study, a new trivalent bispecific nanoantibody has been constructed, which can target both IL5 and HSA. The part targeting IL5 is composed of heterogenous bivalent bisepitope nanoantibody, and the part targeting HSA reaches a long half-life by binding to human albumin. The trivalent IL5-HSA nanoantibody showed extremely strong affinity and its functional activity was significantly better than the commercially available drug mepozumab (monoclonal antibody targeting IL5, Nucala). The antibody exhibits excellent pharmacokinetic characteristics and can continuously reduce the level of eosinophils in peripheral blood. It is expected to support the clinical administration cycle of 2-3 months and greatly improve the drug compliance of patients. In addition, the trivalent IL5-HSA nanoantibody can be produced on a large scale in Pichia pastoris system, with good stability and low production cost. The development of this product will show unique advantages in the aspects of efficacy, safety, technology and cost, and has broad application prospects.
About Loki biological albumin nanoantibody long-acting platform
Loqi Biological has developed a long-acting platform of albumin nanoantibody, and specifically developed camel antibody that combines with human serum albumin. This series of antibodies can extend the half-life by combining with human serum albumin, and because of its small molecular weight, it can avoid the adverse effects of HSA, Fc and other fusion proteins on activity, process and efficacy. Moreover, the humanized HSA-specific single-domain antibody and its antibody combination can also be produced in the Pichia pastoris expression system, which greatly reduces the cost of medication, and has great potential as a long-term and high-dose treatment drug for chronic diseases. This platform has great clinical value.
At present, Rocky Biology has many new drug product chains of nanoantibodies, covering respiratory diseases, autoimmune diseases, tumors and other fields. The unique innovative inhalation macromolecular drug research and development platform of LUKIBIO covers asthma, COPD, COVID-19 and other respiratory diseases; The drug directly reaches the focus, with high utilization rate and low toxicity and side effects; Good portability with small atomizer. The first product of the platform, LQ036, the world's first inhaled macromolecular asthma treatment drug, is carrying out phase I clinical research in Australia and China, and human experiments show excellent safety. In addition, the second product of the platform, LQ043, has also been officially submitted to the domestic clinical phase I IND.



