

Share the latest information



As the characteristics of COVID-19 gradually mutate in the direction of decreasing pathogenicity and increasing infection rate, the team of Wan Yakun from Shanghai Luoqi Biology Group, in cooperation with the team of Yang Zhi and Zhu Hua from Peking University Cancer Hospital, based on the existing research on neutralizing nano antibodies, and with the unique advantages of molecular imaging, has developed a new neutralizing nano antibody probe targeting COVID-19 in order to monitor the infection site of COVID-19 in real time, It provides accurate tracing for the evaluation of nano-antibodies and virus residues in vivo.
The study was published in the journal, entitled "Evaluation of SARS-CoV-2-neutralizing VHH antibody Using Virus Receiver Binding Domain Administered Model Micen" (IF=11.036). The first authors of this article are Liu Song, Ding Lei, and Li Guanghui of Novamab, Cancer Hospital of Peking University. Corresponding authors are Dr. Wan Yakun of Novamab, Yang Zhi, researcher Zhu Hua of Peking University Cancer Hospital and Professor Han Hongbin.

COVID-19 caused by SARS-CoV-2 is sweeping the world, and is still in Manyan, continuing to threaten public health. The main challenge of COVID-19 epidemic is the lack of rapid response and efficient methods to identify the pathogen of SARS-CoV-2 virus. For the need of treatment strategies and protective measures, it is urgent to develop additional diagnostic tools to further analyze the disease.
The development of safe and effective new mechanism products to meet the clinical needs is the direction that Loki Biology has been pursuing. In the face of the wanton epidemic, Luoqi Biological took the lead in arranging the research on the neutralizing antibody of SARS-COV-2 COVID-19. As an effective means to prevent and treat COVID-19 infection, the ND50 of COVID-19 neutralizing nano antibody developed by Luoqi Biological can reach 0.55 μ g/ml (MedComm, 2021). In the process of SARS CoV-2 infection, The drug can effectively prevent the receptor binding domain (RBD) of spike protein S1 subunit of SARS CoV-2 from binding to angiotensin converting enzyme 2 (ACE2) in human body, and play the therapeutic role of COVID-19 by blocking the fusion of virus and cell membrane.
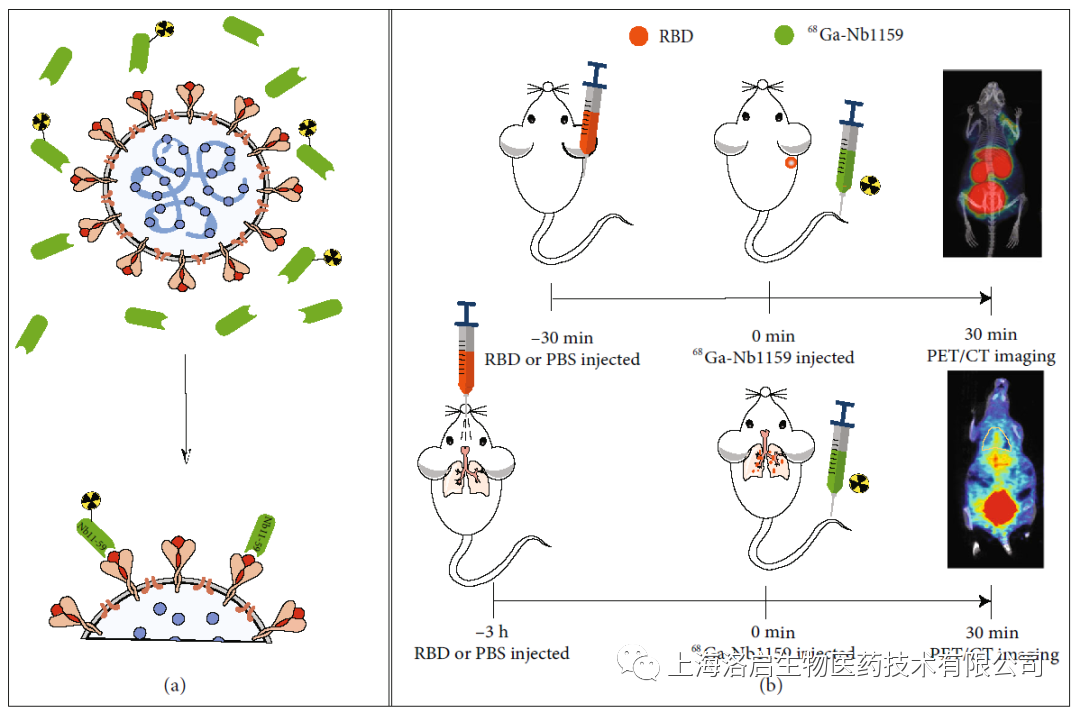
This study is based on the fact that the neutralizing nanoantibody Nb11-59 can bind to SARS-CoV-2 RBD site. It can neutralize the virus and prevent the infection of the virus in the organism by blocking the binding process of SARS-CoV-2 RBD and human ACE2. On the other hand, compared with intact antibodies, nanoantibodies have the advantages of small size, high specificity and stable properties, and have great development potential in production and clinical application. The probe targeting SARS-CoV-2 RBD based on neutralizing antibody is one of the effective means for real-time detection of viral infection sites in physical examination, and plays a role in monitoring the efficacy of other therapeutic drugs.
Through PET technology evaluation, neutralizing nanoantibodies have the following characteristics:
1)A non-invasive and highly sensitive visual imaging method;
2)Provide real-time information on the dynamic state of the whole organism;
3) It is expected to monitor and even predict the response of organisms to treatment or vaccine.
PET technology will become a powerful tool to evaluate the new SARS-CoV-2 and nano bodies and detect their ability to target RBD in vivo.
The deputy editor of Research magazine published an editorial entitled "Fixing the SARS-CoV-2 panel: focusing a new lens on COVID-19" for this article, proposing that this study "is expected to be used to determine the RBD residue after organism infection, evaluate the therapeutic effect of other neutralizing nanoantibodies, and guide the precise treatment during infection".
[1] Song Liu†, Guanghui Li†, Lei Ding†, Jin Ding, Qian Zhang, Dan Li, Xingguo Hou, Xiangxing Kong, Jing Zou, Shiming Zhang, Hongbin Han*, Yakun Wan*, Zhi Yang*, Hua Zhu*. Evaluation of SARS-CoV-2-neutralizing VHH antibody Using Virus Receptor Binding Domain Administered Model Mice. Research, 2022, In press.
END



